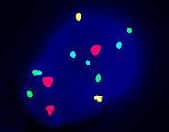
Bladder Cancer Screen by Fluorescence in situ Hybridization
The Bladder Cancer Kit is designed to detect aneuploidy for chromosomes 3, 7, and 17 and loss or deletion of the 9p21 locus via fluorescence in situ hybridization (FISH).
The test is performed on urine specimens from individuals with hematuria or atypical cell morphology suspected of having bladder cancer or on urine from patients previously diagnosed with bladder cancer.
Bladder Cancer testing is a non-invasive method to aid in the initial diagnosis of bladder cancer and in the early detection of tumor recurrence in patients previously diagnosed with bladder cancer. The results of the Bladder Cancer assay should be interpreted in conjunction with standard diagnostic procedures.
Advantages of Bladder Cancer Testing
The Bladder Cancer Kit enumerates specific chromosome regions to detect cancer-associated genetic changes.
Testing provides information that is complementary to results obtained by cystoscopy.
Direct analysis of cytogenetic changes reduces subjectivity in the interpretation of test results.
Less subjectivity affords more accurate monitoring of bladder cancer.
More accurate monitoring provides more information for therapeutic decisions.
The first molecular cytogenetic test for monitoring the recurrence of bladder cancer. Utilizes DNA probes and fluorescence DNA probe technology.
Non-invasive – Cells for analysis are harvested from voided urine. As few as four cells with certain genetic changes identified by the Kit can indicate bladder cancer recurrence.
Highly sensitive for detecting higher stage, higher grade bladder cancer tumors (100% sensitive for stage T2 and Tis tumors). The Kit also detects lower-stage, low-grade tumors (94% sensitive for grade 3 tumors). High sensitivity means few false negatives.
Highly specific for cancer cells. In 275 patients (59 normal volunteers and 216 non-healthy subjects), the Kit showed 94.5% specificity. In healthy volunteers (smokers and non-smokers over age 50), the specificity was 100%. High specificity means fewer false positive results.
Results unaffected by Bacillus Calmette-Guérin (BCG) intravesical therapy.
Readily reimbursed by private and public payers. Bladder cancer testing detects abnormalities in chromosome copy numbers that can occur independently of significant changes in cell morphology, making it more sensitive than conventional urine cytology. Both tests look for cancer cells shed into the urine, but the Bladder Cancer Kit identifies potential cancer cells by detecting specific cytogenetic changes.
Bladder Cancer testing helps identify the presence of the following:
- in situ tumors that lie flat against the bladder wall
- Transitional Cell Carcinomas (TCCs)
- Suspected tumor sites in patients receiving BCG immunotherapy (Often, the inflammation caused by BCG immunotherapy may mask or make it difficult to visualize a tumor. Bladder Cancer testing can help clinicians determine whether or not a site is cancerous.)
- Other urinary tract-related cancers beyond the view of the cystoscope, including ureter, urethra, renal, or prostate, indicate that additional follow-up is warranted.
One Source
Coordinating laboratory tests and results within one facility, MPLN provides a single source for anatomic pathology, flow cytometry, cytogenetics, FISH, and molecular testing. Using one source for your laboratory testing provides simpler logistics for ordering, downstream testing, reporting, billing, and patient management.








